Aromas and flavor: Many drinkers don’t understand how the vanilla, honey, citrus, leather, and wood got into their spirit glass, or whiskey (or other spirits) and some believe they’re added after distillation. Not true. Heat and chemical interactions of fermentation and distillation create the same odorous compounds in spirits found naturally in vanilla beans, honey, oranges, leather, and oak. Read, learn, don’t assume; checking sources ensures myth-free education.
Informative evaluation depends on getting all aromas to the nose. Understanding evaporation and how it’s affected by glassware is key to enjoying spirits, performing meaningful evaluations, and choosing the most important spirits diagnostic equipment, the spirits glass. WARNING: The discussion gets science here and perseverance will be rewarded with useful knowledge. If “molecule” scares you, run now, run fast. Understanding the basic science is key to the focus of becoming a good evaluator.
What is in a 40% ABV spirit? Mostly water (58%), and ethanol alcohol (40%), and the rest, a generous 2% are compounds that give the spirit its character aromas that the nose can detect and the brain identifies as scotch, bourbon, tequila, rum, etc. However, that mix is not what is available for detection at the nose.
What aromas are available at the nose in a 40% ABV Spirit? Ethanol alcohol evaporates much faster than water, so aroma molecules in the evaporation cloud near the glass rim can easily contain 95% ethanol, 2-3% water, and 2- 3% character aromas, mixed in with the normal molecules of the atmosphere. Tiny amounts of character aromas are sufficient to identify a spirit because the human nose can detect and identify very small amounts. The task in evaluating spirits is to catch and identify as many character aromas as possible before the sniffer goes numb from overabundant ethanol (see earlier presentation).
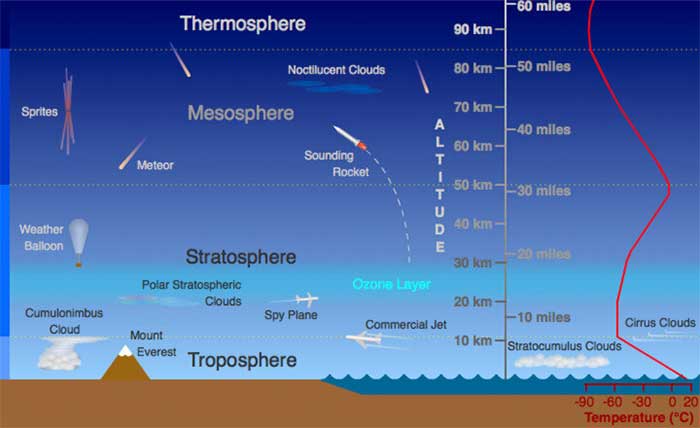
Where do aromas go when they leave the glass? Many believe that evaporated aroma compounds rise high up and disappear into the atmosphere, but exactly the opposite is true. At sea level, air is 78% nitrogen, 21% oxygen, average mass of 29. Weight is mass attracted by gravity, smaller mass = lighter weight. Macro-stratification of the atmosphere is a law of nature. Gravity holds molecules close to the earth, in layers (strata) of molecular mass, heaviest on the bottom, yet partially mixed by weather conditions created by the earth’s rotation and seasonal tilt of the axis, the winds of temperature changes, and land mass cooling, moon gravity pull and tides, ice cap snow melt, solar winds, etc. (macro-weather).
As we leave the earth, fewer molecules are available; oxygen concentration decreases from 29% to 6.8% at the peak of Everest, to 0% higher up. Molecules heavier than air at sea level (29 mass), which are not disturbed by the air currents of weather end up on the ground. Most heavy aroma molecules in the atmosphere disappear from detection quickly at only 20 feet above the ground.
Experiment: drop a piece of dry ice (frozen CO2, mass 44) into a tumbler half-filled with water (H20 mass 18). Dry ice sublimates (changes directly from a solid to a gas without a liquid state) as it is quickly boiled (boiling point of CO2 is -700F) by warm water. The visible mini-ice crystals are clumps of frozen water molecules and carbon dioxide molecules that are carried out of the glass by the increased vapor pressure of sublimation, and fall down the outside of the glass in a visual simulation that accurately describes the movement of unseen, heavier-than-air aroma molecules as they leave a spirits glass
Laws of Nature: At rest and undisturbed, natural law has a profound way of emptying your glass as it sits on the kitchen counter. The vapor pressure of evaporation slowly pushes molecules up and out of the glass where they disperse into the atmosphere, are carried elsewhere by air currents, or settle to the countertop, where some remain, to be picked up by the cleaning sponge, and others are blown over the edge of the counter as gravity pulls them to the floor. Aroma molecules are heavier than air and do not disappear into the upper atmosphere.

Mini-stratification of aromas in the glass is also natural law – until human intervention occurs: Molecular stratification also occurs in the mini-weather cell environment of your spirits glass. Ethanol (46.7 mass) is the smallest (lightest) mass compound in the spirit and the first to evaporate (most volatile). One exception is lower mass water (18.02) evaporates much slower than ethanol even though it is lighter because water molecules are “attracted” to each other, held together by a hydrogen bond which must be broken to release them for evaporation.
Experiment: Smell and taste a partially empty glass of straight whiskey left to stand overnight. The alcohol is gone, but some water remains, as does a stronger smell of character aromas.
Imagine you are riding on a smoky phenol molecule evaporating into the headspace (below the rim, above the liquid surface) of a glass of scotch. You are tossed around and beaten severely in the violent weather of molecular bombardment, collisions, and ethanol windstorms occurring in the glass. It is micro-weather in the glass. Once ejected by vapor pressure your steed (phenol molecule) falls to the ground, and eventually decays into its primary elements of carbon, hydrogen, and oxygen. Micro-weather in the glass can be human-controlled by glass shape, swirling, and covering samples before nosing, all to get more aromas to the nose for detection.
The closer the nose to the spirit, the more aromas to detect: At rest, many large mass aromas lurk low in the glass, mostly out of reach of the inquisitive nose, with a loose stratification similar to the atmosphere. A select few will manage to reach the nostrils because; (1) vapor pressure originating at the liquid surface continually pushes them toward the rim, (2) the evaporation of overabundant ethanol creates currents in the headspace, mixing colliding molecules in the “micro-storm” and carrying aromas to the rim for detection. (3) vigorous swirling violently ejects liquid aromas into the headspace, and (4) nasal inhalation will suck them up.
Volatile ethanol creates “weather in the glass” which aids in bringing aromas to the nose to smell. Micro-storm intensity increases to mini-hurricane Force 10 by swirling. At rest, aromas tend to stratify or fall back into the spirit. That’s the semi-scientific-meteorological discussion of aroma transfer to the rim of the glass, and a takeaway reminder: Takeaway: Always swirl to get more aromas to the nose and choose a glass that gets your nose closer to the surface of the liquid and provides sufficient headspace to allow the evap- cloud to expand. Compare different glasses side-by-side.
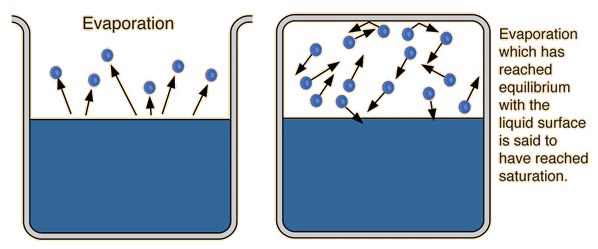
Evaporation in closed containers: Evaporation occurs differently in covered glass, as aroma molecules saturate the airspace (headspace) and none can evaporate until some are re-absorbed by the liquid. This state of equilibrium is ideal because it releases more aromas for detection. Takeaway: Always cover evaluation samples until ready to smell. Swirling while covered breaks surface tension, releases aromas, and hastens equilibrium. Removing the cover, aroma molecules diffuse in two ways:
(1) molecular diffusion from high concentration to low concentration due to thermal motion (heat or kinetic energy), and (2) eddy diffusion from turbulent mixing (weather in the glass from volatile ethanol currents and swirling). Molecular diffusion can be controlled by glassware bowl and rim shape. Common glassware manufacturers, unconcerned with physical science, consistently ignore this great opportunity to produce functional glassware to provide better aroma delivery and detection.
Revisiting the importance of ortho-nasal aroma detection: In a previous presentation, the differences between ortho-nasal (smell alone, through the nostrils) and retro-nasal (taste, smell, and mouthfeel together, through the oral cavity and pharyngeal opening at the back of the throat) were discussed. The science of olfactory and effective spirits evaluation is much more complicated than detecting smells; it involves all the senses and emotions to imprint a more complete perception and mentally catalog aromas in your smell library for future reference.
We noted previously that visualizing the experience during ortho-nasal smelling before tasting gives a moment to pause and create a multi-sensual visual-picture-emotional association with each aroma when adding it to our smell- libraries and bringing it into more definitive focus at later recall. This moment also provides an opportunity to compare the olfactory-only perception to the trigeminal retro-nasal finish evaluation, rounding out aroma perception from two distinctly different aspects.
Memory constantly builds our smell libraries, and we tend to group similar aromas (is the smell in my gin anise, chervil, tarragon or fennel). Ortho-nasal sets the tone for recalling these aromas as well as their related aroma families, preparing the evaluator for what is to come, whereas retro-nasal relies on the effect of oral cavity warming, sloshing the spirit around inside the oral cavity, conditioning with saliva, and passage of released aromas through the pharyngeal opening to enter the olfactory cavity for detection.

Contrary to the tongue-map myth perpetrated by wine-glass makers, retro-nasal detection is not affected by glass style or shape, as it takes place entirely within the oral cavity and posterior olfactory cavities. Since delivery into the mouth has no aroma or taste effect, vessel shape is not remotely a retro-nasal evaluation factor. Retro-nasal is necessary for accurate evaluation (it is the finish attribute) but by itself, may not jog the memory to recall the complete visual and emotional picture of aroma and flavor. Takeaway: Choose spirits glasses that present a complete ortho-nasal aroma display, to set the stage for effective evaluation and smell-library cataloging. Utilize ortho-nasal and retro-nasal together to complete aroma and flavor profiles. Stay away from cons and “seminars” involving flavor presentation due to the glass shape.
The Aroma Cloud and Effective Nose Positioning for the Three Common Spirits Glasses:
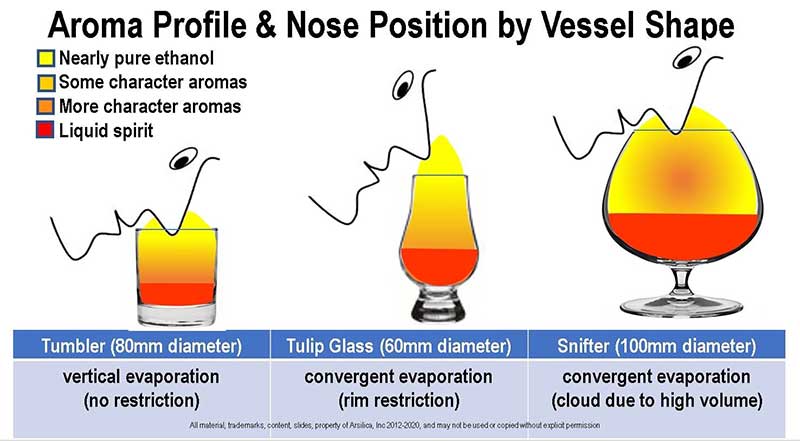
- Tumbler: Vertical sides, aroma manipulation possible only by swirling. The tumbler is easy to swirl, and the large rim accommodates a large evap-cloud extending slightly above the rim. More character aromas are found if the nose is placed in the glass below the rim, but more ethanol is there as well. The tumbler is the best glass of the three common types for detecting character aromas with much less ethanol than the other two basic shapes discussed here. Tilting the glass tends to release an invisible tidal wave of evaporated aromas toward the nostrils, and if aided by slight nasal inhalation, provides an opportunity for a thoughtful sniff.
- Tulip and Copita: The favorite glass of whiskey drinkers worldwide, the long slender shape and small bowl diameter don’t swirl as easily as a tumbler and the tiny rim prevents the nose from entering the glass. Instead, the nose contacts the upper rim of the glass (see exhibit) and is kept outside the aroma cloud. The aroma cloud is somewhat stratified in the glass, and larger character aromas are stratified in the lower bowl, concentrated ethanol rising to the rim. Swirling releases some character aromas, but also releases more ethanol. Ethanol is significantly prevalent, concentrated at the rim, and ortho-nasal sniffing to pick up aromas before ethanol numbs the olfactory is a daunting, random process. In other words, locating character aromas is a guessing game, and nose-numbing ethanol anesthesia sets in quickly as high concentrations of ethanol take their toll. Useful aroma detection from tulip-shaped glasses occurs retro-nasally after the spirit has been sloshed around in the mouth and conditioned with saliva, negating the importance of glass shape, as discussed.
- Snifter: The large bowl and tiny rim demand the nose be inserted directly into the bowl to find character aromas. The snifter is hands down the best swirled (earlier swirling exhibit re-presented below), but sadly, it’s also a collector and concentrator of ethanol which obscures character aromas. There is simply no way to separate character from nose-numbing ethanol in a snifter. Truly an ethanol nose bomb, and quickest to numb the olfactory since the nose must be inserted into the glass, snifters should be avoided altogether. More snifter pitfalls in a later presentation.
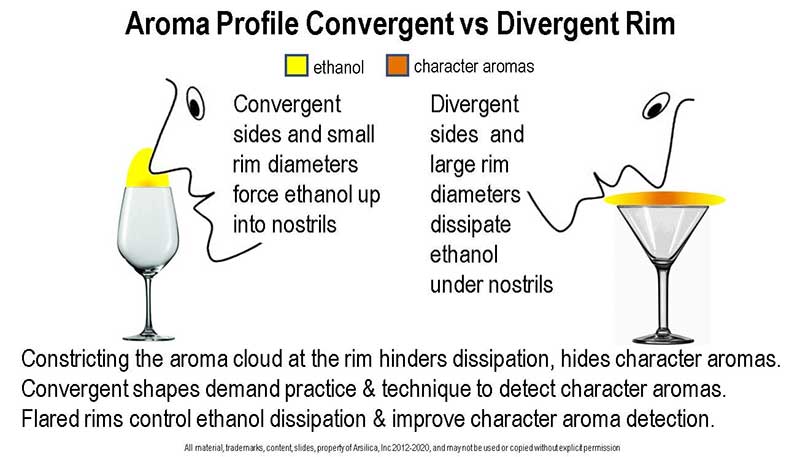
Vessel Rim Shape Affects Ethanol Concentration in Spirits Glasses: The diagram shows the difference between convergent and divergent rims. In a future presentation, we will explore the manipulation of the aroma cloud to eliminate ethanol anesthesia through rim shape and size and provide a complete evaluation of all three common glass shapes.
Takeaway: Aroma detection is all about evaporation, swirling, and shape-influenced aroma-cloud presentation. Different glasses swirl differently and present detectable aromas to the nose differently. Aroma identification is hindered by ethanol, the overabundant, anesthetic enemy. Difficulty in avoiding nose-numbing ethanol demands care and awareness when using commonly accepted, traditional glass shapes and styles for nosing and evaluating spirits, as character aromas are masked and hidden behind strong ethanol concentrations. See the major competitions that use the NEAT glass as their official judging glass. Independent research validates our findings.







